
Päivi M. Ojala
Ph.D., Professor in Cancer Cell Biology & K. Albin Johansson Research Professor
Research Programs Unit, Translational Cancer Biology Medicum Faculty of Medicine Biomedicum Helsinki
P.O. Box 63 (Haartmaninkatu 8) FI-00014 University of Helsinki Finland
Tel. +358 2 941 59445 Fax +358 2 941 25610
firstname.surname@helsinki.fi
Affiliations
- Research Programs Unit
- Translational Cancer Biology Research Program
- Centre of Excellence in Translational Cancer Biology
- Medicum


Ojala lab
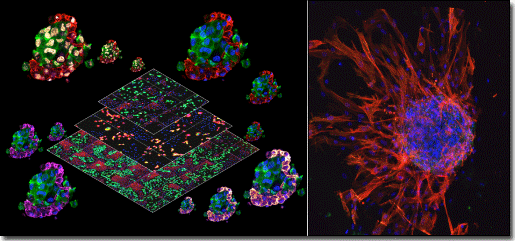
Our research program is focused to make fundamental contributions to the fields of cancer biology, tumor microenvironment and virus-host cell interactions. Research on human tumor viruses has been central to development of modern cancer biology and given profound insights into not only cancers associated with infection but also cancer in general. We are using Kaposi’s sarcoma herpesvirus (KSHV), an oncogenic gamma-2 herpesvirus, as a model system to study the virus-host interactions. Our previous contributions include demonstration of p53 reactivation as a novel therapeutic modality for KSHV associated lymphomas and reports on viral oncogene-induced DNA damage and defects in T cell differentiation and lymphoma initiation in vivo.
The switch between the latency and lytic replication (virus reactivation) is a critical step in KSHV pathogenesis, and our research is also focused to identify novel signaling pathways and cellular proteins involved in viral reactivation by functional genomics approaches. Our previous and recent work has identified cellular Pim kinases, nucleophosmin (NPM) and the p53-p21 axis as novel regulators important for viral reactivation.
We have recently developed a novel 3D organotypic cell model for KSHV-infected primary lymphatic endothelial cells (LECs). These studies led to a significant discovery demonstrating that KSHV can transcriptionally reprogram LECs to a mesenchymal and invasive cell type via endothelial-to-mesenchymal transition (EndMT).
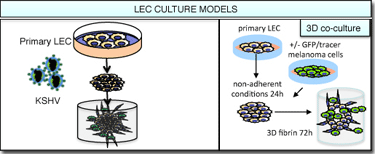 The discovery of virus-induced cellular reprogramming has motivated us to hypothesize that changes in the LEC identity and function could be involved also in other cancers involving interaction with the lymphatics. We are interested in understanding the molecular circuits contributing to melanoma metastasis. Malignant melanomas disseminate primarily through the lymphatic system, and therefore the project focuses on the interactions of melanoma cells with the lymphatic endothelial cells (LECs). We have developed several co-culture cell models to mimic the melanoma-LEC interactions in vivo, and found intriguing changes in both the cancer cells and LECs. We are currently addressing the contribution of these changes to melanoma progression and metastasis.
The discovery of virus-induced cellular reprogramming has motivated us to hypothesize that changes in the LEC identity and function could be involved also in other cancers involving interaction with the lymphatics. We are interested in understanding the molecular circuits contributing to melanoma metastasis. Malignant melanomas disseminate primarily through the lymphatic system, and therefore the project focuses on the interactions of melanoma cells with the lymphatic endothelial cells (LECs). We have developed several co-culture cell models to mimic the melanoma-LEC interactions in vivo, and found intriguing changes in both the cancer cells and LECs. We are currently addressing the contribution of these changes to melanoma progression and metastasis.
