|
Contact Information: Andrey Anisimov Postal address: |
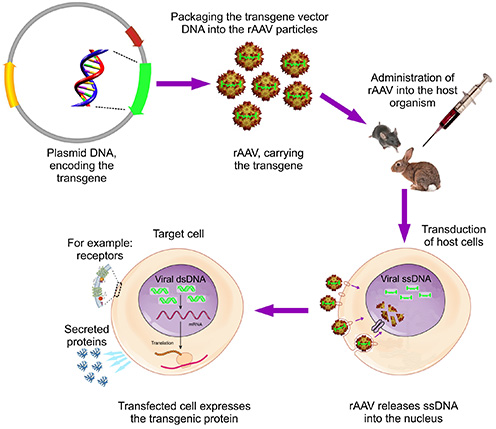
The Biomedicum AAV Core Facility provides services for manufacturing recombinant adeno-associated virus (AAV) preps from the customer’s gene-encoding plasmids. AAVs are able to transduce both dividing and non-dividing cells, does not provoke insertional mutagenesis and possesses very low immunogenicity. Currently we produce AAVs of 4 different serotypes (AAV-2, 6, 8 and 9). Final virus preps are quantified and functionally characterized for the transgene expression (normally by western blotting, ELISA or fluorescent signal detection). There are several ready AAV preps, immediately available on demand. These encode EGFP, Luciferase, LacZ, HSA, Fc part of mouse IgG, various growth factors and soluble receptors or were produced using an empty plasmid vector for the negative control. Current prices: internal academic and HUS customers: € 676; external academic customers: € 1050 and external non-academic customers: € 1397. We also consult the customers (free of charge) for the optimal design of AAV vectors, which would carry the customer’s transgene.
Page updated December 12, 2017 |
|