| Affiliations:
Contact Information: Petri Salven |
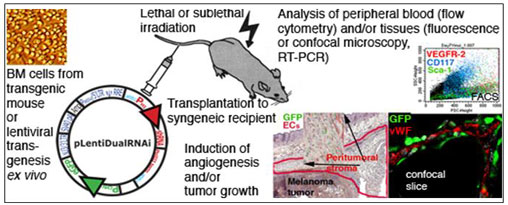
Background: Salven groups's website has moved. http://www.hi.helsinki.fi/english/research/pathology.html Novel, still controversial experimental data indicate that adult bone marrow (BM) stem and progenitor cells may differentiate to various non-hematopoietic cell and tissue types. Therefore, adult bone marrow provides a potential source for stem cells that could be used in therapeutic applications such as cell and tissue regeneration and repair. Autologous stem cells and their progeny would be histocompatible with the patient's other cells, and be expected to have a normal life span. Additionally, adult stem cells could be used as organ/tissue type-specific vehicles for anticancer or other therapeutic agents or gene therapy vectors when targeting specific tissues, tumors, or tumor blood vessels. The tumor microenvironment consists of cells of hematopoietic and mesenchymal origin, including stem and progenitor cells, inflammatory cells, fibroblasts, endothelial cells, and other vascular mural cells. Tumor growth is known to depend on the interaction of tumor cells with such stromal cells. For example, a growing tumor needs to recruit normal endothelial and vascular mural cells to form its blood vessels. Increasing data indicate that the BM contributes different cell types to the tumor stroma, and that these cells may regulate tumor angiogenesis and growth. However, the functional relevance of the different BM-derived cell types and their interaction with tumor cells and other stromal cells remains to be established. Stromal cell-dependent interactions represent an attractive target for cancer therapy, because normal cells are genetically stable, and they would not be expected to develop resistance to therapeutic agents . The development of such therapies is hampered by the fact that the molecular mechanisms behind tumor-stroma interactions are often poorly understood. Our work aims to an understanding of these questions at the molecular level, and should unravel new targets for anti-angiogenic and/or anti-tumor therapies, and new strategies for delivering therapy to tumors. Current focus areas are: High throughput differentiation screening of established murine and human embryonic stem (ES) cell lines. High throughput functional genomics methods are utilized to induce differentiation of marker-gene tagged ES cells to mature cell and/or tissue types of biological and medical importance, and to analyze the molecular machinery governing stem cell self-renewal. The ES cell differentiation screens are carried out using cDNA, RNAi and chemical libraries in close collaboration with the Taipale Lab and the Biomedicum High Throughput Center. Molecular machinery governing adult stem cell self-renewal and differentiation. Stem cells are defined as cells that have the ability to perpetuate themselves through self-renewal and to generate mature cells of a particular tissue through differentiation. To identify signaling pathways essential for adult stem cell self-renewal, growth, or differentiation, we use tailored lentiviral libraries to target selected central cell signaling pathways in BM stem cells, and study the effects of the affected pathways in vivo in mice. Stem and progenitor cells contributing to angiogenesis. Recruitment of BM-derived endothelial precursors has been argued to be both necessary and sufficient for tumor angiogenesis and growth, suggesting that inhibition of the function of these EC precursors might provide a novel approach for blocking tumor growth. However, our recent publications show that during tumor growth, progenitors for periendothelial vascular mural cells are mobilized from the BM, whereas we found no evidence of their incorporation into growing blood vascular or lymphatic endothelia. We now continue the characterization of these newly found BM-derived periendothelial progenitors in mouse models of tumor- or cytokine-induced angiogenesis. We use chimeric animals created by reconstituting the wild type BM with stem cells expressing reporter genes (GFP, ß-galactosidase) under universal or specific promoters. Factors regulating the differentiation of BM-derived stem cells to vascular mural cells are identified using functional genomic analyses and in vivo angiogenesis and tumor models.
Dissection of the functions of selected BM/endothelial/stromal cell specific signaling pathways. BM cells have been reported to contribute to multiple cell populations in tumor stroma, including endothelial cells, periendothelial cells and hematopoietic effector cells. To more clearly define the BM derived cell populations that are critical in promotion of angiogenesis and tumor growth, and to identify the crucial regulatory pathways involved in these processes, we will specifically analyze selected cell-surface receptors and signaling pathways that are thought to regulate BM-derived and/or vascular cells. Commonly, many of these molecules are believed to be phenotypic and/or functional reflections of the embryonic hemangioblast. However, because of the technical difficulties associated with identifying the true adult stem cells, there exists a great deal of controversy regarding such molecules. We are now constructing lentiviral shRNA vectors (pLentiDualRNAi) targeting the selected genes of interest. The effects of the silenced pathways are studied in vivo in mouse models of angiogenesis and cancer. Downstream events resulting from specific inactivation of a targeted gene are also studied using global gene expression analyses.
Page updated February 13, 2015 |
|